| References |
| Synonyms |
- Nuclear Hormone Receptor 1
- NUC1
- FAAR
- PPARβ
|
| Formulation |
Peptide affinity-purified IgG |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| Specificity |
| Human PPARδ |
+ |
| Mouse PPARδ |
+ |
| Ovine PPARδ |
+ |
| Porcine PPARδ |
+ |
| Rat PPARδ |
+ |
Show all 5
Hide all but first 3
|
Background Reading
Dreyer, C., Krey, G., Keller, H., et al. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68 879-887 (1992).
Amri, E., Bonino, F., Ailhaud, G., et al. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem 270 2367-2371 (1995).
Lim, H., and Dey, S.K. PPARδ functions as a prostacyclin receptor in blastocyst implantation. Trends Endocrinol Metab 11(4) 137-142 (2000).
Mammalian Gene Collection (MGC) Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA 99(26) 16899-16903 (2002).
Wang, Y., Lee, C., Tiep, S., et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113 159-170 (2003).
He, T., Chan, T.A., Vogelstein, B., et al. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99 335-345 (1999).
Berger, J., Leibowitz, M.D., Doebber, T.W., et al. Novel peroxisome proliferator-activated receptor (PPAR) γ and PPARδ ligands produce distinct biological effects. J Biol Chem 274 6718-6725 (1999).
Willson, T.M., Brown, P.J., Sternbach, D.D., et al. The PPARs: From orphan receptors to drug discovery. J Med Chem 43(4) 528-550 (2000).
Show all 8
Hide all but first 3
| Size |
Global Purchasing |
| 500 µl |
|
Description
Antigen:
human PPARδ amino acids 39-54 (SSSYTDLSRSSSPPSL)
·
Host:
rabbit
·
Application(s):
WB, IHC, and ICC; other applications not tested
·
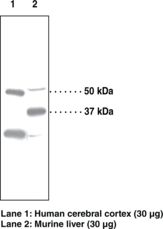
PPARδ is one of three peroxisome proliferator-activated receptor (PPAR) subtypes that possess a domain structure common to other members of the nuclear receptor gene family. It was first cloned from Xenopus laevis and named PPARβ.1 PPARδ is ubiquitously expressed but is particularly abundant in tissues such as liver, intestine, kidney, abdominal adipose, and skeletal muscle, all of which are involved in lipid metabolism.2 PPARδ is a mediator of diverse physiological functions including lipid and cholesterol homeostasis, embryo implantation, and cancer development.3,4,5,6 Most recently, attention has been focused on the role of PPARδ in obesity.7 Cayman’s PPARδ polyclonal antibody can be used for western blot, immunohistochemistry, and immunocytochemistry to study the expression and functions of this protein. The antibody recognizes PPARδ at 50 kDa from human samples. An additional smaller size of PPARδ (~ 40 kDa)8 is also detected in certain mouse tissues.
1
Dreyer, C., Krey, G., Keller, H., et al. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68 879-887 (1992).
2
Willson, T.M., Brown, P.J., Sternbach, D.D., et al. The PPARs: From orphan receptors to drug discovery. J Med Chem 43(4) 528-550 (2000).
3
Amri, E., Bonino, F., Ailhaud, G., et al. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem 270 2367-2371 (1995).
4
Berger, J., Leibowitz, M.D., Doebber, T.W., et al. Novel peroxisome proliferator-activated receptor (PPAR) γ and PPARδ ligands produce distinct biological effects. J Biol Chem 274 6718-6725 (1999).
5
Lim, H., and Dey, S.K. PPARδ functions as a prostacyclin receptor in blastocyst implantation. Trends Endocrinol Metab 11(4) 137-142 (2000).
6
He, T., Chan, T.A., Vogelstein, B., et al. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99 335-345 (1999).
7
Wang, Y., Lee, C., Tiep, S., et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113 159-170 (2003).
8
Mammalian Gene Collection (MGC) Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA 99(26) 16899-16903 (2002).
|