服务热线
021-60498804
产品中心
/ Products Classification 点击展开+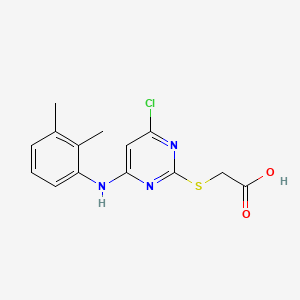
| Cat. Number | 701936324364409 |
|||||||||||||||||||||||||||||||||||||||||||||
| Chemical Name | 吡立尼克酸(Wy 14643) Pirinixic acid |
|||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 50892-23-4 |
|||||||||||||||||||||||||||||||||||||||||||||
| Qty 1 |
10mg |
|||||||||||||||||||||||||||||||||||||||||||||
| Qty 2 | 50mg |
|||||||||||||||||||||||||||||||||||||||||||||
| References | 吡立尼克酸(Wy 14643) Pirinixic acid 是一种天然的、无毒的、无刺激性的羧酸,由于其潜在的治疗应用,在过去的几年里被广泛研究。它是自然界中广泛分布的分子,存在于许多植物、真菌和细菌中,也存在于一些人体组织中。吡立尼克酸具有广泛的生物活性,包括抗炎、抗氧化、抗增殖和抗血管生成特性。它还以其调节各种酶和受体活性的能力而闻名,并因其治疗各种疾病的潜力而被研究,包括癌症、阿尔茨海默病和糖尿病。 订购信息:
产品信息:
合成方法: 吡立尼克酸的合成方法有化学合成和生物转化两种。化学合成是最常用的方法,它涉及到使用各种试剂和催化剂来形成所需的产品。最常见的方法是无水甲酸与氢氧化钠反应生成甲酸钠,甲酸钠再与乙酸等羧酸反应生成吡吡尼酸。生物转化是一种不太常用的方法,涉及使用酶催化底物转化为所需的产品。 科研应用: 吡立尼克酸已经在体内和体外模型中被广泛研究其潜在的治疗应用。体内研究涉及使用动物模型来研究化合物对人体的影响,而体外研究涉及使用细胞培养模型来研究对细胞的影响。 作用机制: 吡吡尼克酸的确切作用机制仍不完全清楚。它被认为是通过调节各种酶和受体的活性起作用,包括COX-2和NF-kB,以及通过诱导癌细胞凋亡。此外,在动物模型中,它已被证明可以抑制肿瘤细胞的生长,减少炎症,并改善认知功能。 生化和生理效应: 吡立尼克酸已被证明可以调节各种酶和受体的活性,包括COX-2和NF-kB。此外,在动物模型中,它已被证明可以减少炎症,改善认知功能,降低血糖水平。 体内研究: 已经证明吡立尼克酸有治疗多种疾病的潜力,包括癌症、阿尔茨海默病和糖尿病。在动物模型中,它已被证明可以抑制肿瘤细胞的生长,减少炎症,并改善认知功能。此外,吡立尼克酸已被证明可以降低糖尿病小鼠的血糖水平。 体外研究: 已经证明吡立尼克酸具有调节各种酶和受体活性的潜力,包括环氧合酶-2 (COX-2)和核因子- κ B (NF-kB)。它还被证明可以抑制多种癌细胞的生长,包括乳腺癌、肺癌和前列腺癌细胞。此外,吡inixacid已被证明可诱导癌细胞凋亡,并抑制血管内皮细胞的增殖。 生物活性: 吡立尼克酸已被证明具有广泛的生物活性,包括抗炎、抗氧化、抗增殖和抗血管生成特性。它还被证明可以调节各种酶和受体的活性,包括COX-2和NF-kB,以及诱导癌细胞凋亡。此外,吡立尼克酸已被证明可以降低糖尿病小鼠的血糖水平。 实验室实验的优势与局限性: 在实验室实验中使用吡立尼克酸的优点包括它的无毒和无刺激性的特性,以及它能够调节各种酶和受体的活性,包括COX-2和NF-kB。此外,它已被证明能诱导癌细胞凋亡,并降低糖尿病小鼠的血糖水平。在实验室实验中使用吡立尼克酸的主要限制是它尚未被批准用于人体,因此其潜在的治疗应用还有待充分开发。 药效学: 吡立尼克酸是一种无毒、无刺激性的羧酸,以其调节各种酶和受体(包括COX-2和NF-kB)活性的能力而闻名。此外,在动物模型中,它已被证明可以抑制肿瘤细胞的生长,减少炎症,并改善认知功能。 未来方向: 吡立尼克酸的潜在治疗应用仍在探索中,未来有许多方向可以探索。这些研究包括进一步研究其作用机制、治疗各种疾病的潜力,以及与其他药物或疗法联合使用的潜力。此外,还可以进一步研究它作为膳食补充剂的潜力,以及它在药物输送系统中的潜力。最后,可以进一步研究其在人体中的安全性和有效性,以及在临床试验中使用的潜力。 上海惠诚生物科技有限公司自2010年成立以来,一直致力于为客户提供试剂,仪器设备和耗材一站式服务。目前在苏州和南通的研发生产基地,专注于体外诊断蛋白,靶标蛋白,工业用酶和细胞因子的生产和定制,按照GMP标准,为工业客户提供大包装蛋白原料。 更多产品请联系惠诚生物! 蛋白原料网站:www.acediag.com 全国统一服务热线:021- 60496554 24小时手机服务: 13917250134(同微信)QQ:1715451510 Email:info@e-biochem.com |
|||||||||||||||||||||||||||||||||||||||||||||





