服务热线
021-60498804
产品中心
/ Products Classification 点击展开+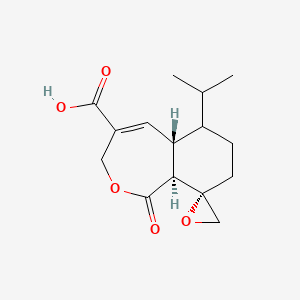
| Cat. Number | HB-167312 |
|||||||||||||||||||||||||||||||||||||||||
| Chemical Name | 七萜酸 Heptelidic acid/Koningic acid |
|||||||||||||||||||||||||||||||||||||||||
| CAS Number | 57710-57-3 |
|||||||||||||||||||||||||||||||||||||||||
| Mol. Formula | C15H20O5 |
|||||||||||||||||||||||||||||||||||||||||
| Mol. Weight | 280.32 |
|||||||||||||||||||||||||||||||||||||||||
| Qty 1 |
1mg |
|||||||||||||||||||||||||||||||||||||||||
| Qty 2 | 5mg |
|||||||||||||||||||||||||||||||||||||||||
| Appearance | White solid. |
|||||||||||||||||||||||||||||||||||||||||
| Application Notes | Koningic acid(KA)能抑制GAPDH活性,导致MG大量产生 |
|||||||||||||||||||||||||||||||||||||||||
| Solubility | Soluble in DMSO (10mg/ml), ethanol (1mg/ml), methanol (1mg/ml) or water (1mg/ml). |
|||||||||||||||||||||||||||||||||||||||||
| Storage condition | -20°C |
|||||||||||||||||||||||||||||||||||||||||
| Stability | Stable for at least 3 years |
|||||||||||||||||||||||||||||||||||||||||
| References | 七萜酸 Heptelidic acid (Koningic acid) 是由真菌T. koningii产生的一种邻半萜内酯,对厌氧菌如拟杆菌具有抗菌活性。它作为一种不可逆的GAPDH抑制剂,与酶活性位点的半胱氨酸-149残基结合。七萜酸是一种倍半萜类抗生素 (antibiotic)。七萜酸通过下调 caspase 从而抑制 Etoposide 诱导的细胞凋亡。七萜酸是一种特异性 GAPDH 抑制剂,IC50 为 90 μM。 订购信息:
产品信息:
作用机制: 甘油醛3-磷酸脱氢酶(GAPDH)是碳水化合物代谢的关键酶,可逆地催化GAP转化为1,3-双磷酸甘油酸和NAD+。七萜酸是一种倍半萜内酯,由真菌T. koningii产生,被证明对厌氧菌(如拟杆菌)具有抗生素活性。它作为一种不可逆的GAPDH抑制剂,在酶的活性位点(Ki = 1.6µM)与半胱氨酸-149残基结合。通过抑制糖酵解途径中ATP的生成,可选择性诱导高糖酵解癌细胞凋亡。七萜酸也是哺乳动物DNA聚合酶β和λ的选择性和竞争性抑制剂,以及DNA聚合酶家族X中的末端脱氧核苷酸转移酶(Kis范围为5.2-9.5µM)。 体内研究: 七萜酸具有生物可利用性,可诱导体内糖酵解的动态变化。 体外研究: 七萜酸是一种倍半萜类抗生素,在从土壤样本中分离出的三种不同真菌菌株的培养滤液中发现。七萜酸生产生物、发酵、分离和表征。七萜酸抑制依泊苷诱导的人白血病U937细胞凋亡。七萜酸抑制了U937细胞caspase- 3的诱导,其IC50值为40 μM。 七萜酸是从木霉真菌中获得的天然产物,可直接与人GAPDH的活性位点结合。kkoningii KAr-GAPDH的表达成功地挽救了Heptelidic酸处理过的人细胞的活力。表达kkoningii ka - gapdh的HEK293T细胞经0 ~ 200 μM七泰酸(IC50=5 μM)处理后具有完全的细胞活力。 上海惠诚生物科技有限公司自2010年成立以来,一直致力于为客户提供试剂,仪器设备和耗材一站式服务。目前在苏州和南通的研发生产基地,专注于体外诊断蛋白,靶标蛋白,工业用酶和细胞因子的生产和定制,按照GMP标准,为工业客户提供大包装蛋白原料。 更多产品请联系惠诚生物! 蛋白原料网站:www.acediag.com 全国统一服务热线:021- 60496554 24小时手机服务: 13917250134(同微信)QQ:1715451510 Email:info@e-biochem.com |
|||||||||||||||||||||||||||||||||||||||||





