服务热线
021-60498804
产品中心
/ Products Classification 点击展开+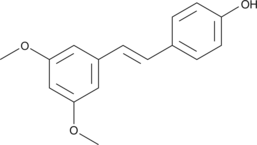
| Cat. Number | 080407161642919 |
||||||||||||||||||||||||||||||||
| Chemical Name | Pterostilbene |
||||||||||||||||||||||||||||||||
| References |
Background ReadingRimando, A.M., Cuendet, M., Desmarchelier, C., et al. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50 3453-3457 (2002). Stivala, L.A., Savio, M., Carafoli, F., et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem 276(25) 22586-22594 (2001). Pan, M., Chang, Y., Tsai, M., et al. Pterostilbene suppressed lipopolysaccharide- Pan, M., Lin, Y., Lin, C., et al. Suppression of heregulin- Hougee, S., Faber, J., Sanders, A., et al. Selective COX- Pan, M., Chiou, Y., Chen, W., et al. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis 30(7) 1234-1242 (2009). Show all 6 Hide all but first 3
Description
Pterostilbene is a naturally-
1 Rimando, A.M., Cuendet, M., Desmarchelier, C., et al. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50 3453-3457 (2002). 2 Stivala, L.A., Savio, M., Carafoli, F., et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem 276(25) 22586-22594 (2001).
3
Hougee, S., Faber, J., Sanders, A., et al. Selective COX-
4
Pan, M., Chang, Y., Tsai, M., et al. Pterostilbene suppressed lipopolysaccharide-
5
Pan, M., Lin, Y., Lin, C., et al. Suppression of heregulin- 6 Pan, M., Chiou, Y., Chen, W., et al. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis 30(7) 1234-1242 (2009). |
||||||||||||||||||||||||||||||||
下一个:Mn(III)TMPyP上一个:Quercetin |
|||||||||||||||||||||||||||||||||





