| References |
| Synonyms |
- (E)-5-[2-(4-hydroxyphenyl)ethenyl]-1,3-benzene diol-d4
|
| Formal Name |
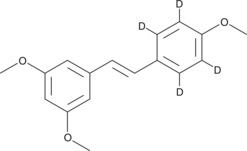
1,3-dimethoxy-5-[(1E)-2-(4-methoxyphenyl)ethenyl]-benzene-2,3,5,6-d4 |
| Molecular Formula |
C17H14D4O3 |
| Formula Weight |
274.4 |
| Formulation |
A solution in methyl acetate |
| Purity |
≥99% deuterated product |
| Stability |
6 months |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
COc1ccc(cc1)\C=C/c1cc(OC)cc(OC)c1
|
Background Reading
Pettit, G.R., Grealish, M.P., Jung, M.K., et al. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate. J Med Chem 45 2534-2542 (2002).
Miller, N.J., and Rice-Evans, C. Antioxidant activity of resveratrol in red wine. Clin Chem 41 1789 (1995).
Frémont, L., Belguendouz, L., and Delpal, S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci 64 2511-2521 (1999).
Johnson, J.L., and Maddipati, K.R. Paradoxical effects of resveratrol on the two prostaglandin H synthases. Prostaglandins Other Lipid Mediat 56 131-143 (1998).
Nam, K.A., Kim, S., Heo, Y.H., et al. Resveratrol analog, 3,5,2',4'-tetramethoxy-trans-stilbene, potentiates the inhibition of cell growth and induces apoptosis in human cancer cells. Arch Pharm Res 24(5) 441-445 (2001).
Show all 5
Hide all but first 3
| Size |
Global Purchasing |
| 100 µg |
|
| 250 µg |
|
| 500 µg |
|
| 1 mg |
|
Description
trans-trismethoxy Resveratrol-d4 contains four deuterium atoms at the 2, 3, 5, and 6 positions. It is intended for use as an internal standard for the quantification of trans-trismethoxy resveratrol by GC- or LC-mass spectrometry. Phenolic compounds, particularly flavonoids, from plant sources have long been observed to have antioxidant activity with potential benefits for human health.1,2,3 Resveratrol is a potent phenolic antioxidant found in grapes and red wine that also has antiproliferative and anti-inflammatory activity.4 When the three phenolic hydroxyl groups of resveratrol are converted to methyl ethers, the inhibition of cell growth and pro-apoptotic activities of resveratrol are enhanced.5
1
Frémont, L., Belguendouz, L., and Delpal, S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci 64 2511-2521 (1999).
2
Johnson, J.L., and Maddipati, K.R. Paradoxical effects of resveratrol on the two prostaglandin H synthases. Prostaglandins Other Lipid Mediat 56 131-143 (1998).
3
Miller, N.J., and Rice-Evans, C. Antioxidant activity of resveratrol in red wine. Clin Chem 41 1789 (1995).
4
Rotondo, S., Rajtar, G., Manarini, S., et al. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol 123 1691-1699 (1998).
5
Nam, K.A., Kim, S., Heo, Y.H., et al. Resveratrol analog, 3,5,2',4'-tetramethoxy-trans-stilbene, potentiates the inhibition of cell growth and induces apoptosis in human cancer cells. Arch Pharm Res 24(5) 441-445 (2001).
|