| References |
| Formal Name |
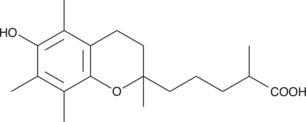
3,4-dihydro-6-hydroxy-α,2,5,7,8-pentamethyl-2H-1-benzopyran-2-pentanoic acid |
| CAS Number |
7083-09-2 |
| Molecular Formula |
C19H28O4 |
| Formula Weight |
320.4 |
| Formulation |
A crystalline solid |
| Purity |
>98% |
| λmax |
210, 292 nm |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
OC(=O)C(C)CCCC1(C)CCc2c(O1)c(C)c(C)c(O)c2C
|
Background Reading
Sontag, T.J., and Parker, R.S. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem 277(28) 25290-25296 (2002).
Pope, S.A.S., Burtin, G.E., Clayton, P.T., et al. New synthesis of (±)-α-CMBHC and its confirmation as a metabolite of α-Tocopherol (vitamin E). Bioorg Med Chem 9 1337-1343 (2001).
Traber, M.G., Elsner, A., and Brigelius-Flohé, R. Synthetic as compared with natural vitamin E is preferentially excreted as α-CEHC in human urine: Studies using deuterated α-tocopheryl acetates. FEBS Lett 437 145-148 (1998).
Birringer, M., Drogan, D., and Brigelius-Flohé, R. Tocopherols are metabolized in HepG2 cells by side chain ω-oxidation and consecutive β-oxidation. Free Radic Biol Med 31(2) 226-232 (2001).
Kamal-Eldin, A., and Appelqvist, L. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31 671-701 (1996).
Brigelius-Flohé, R., and Traber, M.G. Vitamin E: Function and metabolism. FASEB J 13 1145-1155 (1999).
Show all 6
Hide all but first 3
| Size |
Global Purchasing |
| 1 mg |
|
| 5 mg |
|
| 10 mg |
|
| 25 mg |
|
Description
Vitamin E is a well known antioxidant that exists in eight natural forms: four tocopherols (α, β, δ, and γ) and four tocotrienols (α, β, δ, and γ). α-Tocopherol is the major lipid soluble antioxidant in vivo and protects against lipid peroxidation.1,2 α-CEHC is the major urinary metabolite of α-tocopherol following vitamin E supplementation.3 α-CMBHC is the longer side-chain precursor of α-CEHC and a minor metabolite of α-tocopherol.4,5 Hep-G2 cells release α-CEHC, α-CMBHC, and α-CMHHC into the medium when treated with all rac α-tocopherol. Pretreatment of Hep-G2 cells with rifampicin, an inducer of the CYP3A family of cytochrome P450s, results in a 5-fold increase in α-CEHC. This indicates that metabolism of α-tocopherol proceeds by initial ω-oxidation to precursors of α-CMBHC followed by β-oxidation to shorter chain metabolites such as α-CEHC.6
1
Kamal-Eldin, A., and Appelqvist, L. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31 671-701 (1996).
2
Brigelius-Flohé, R., and Traber, M.G. Vitamin E: Function and metabolism. FASEB J 13 1145-1155 (1999).
3
Traber, M.G., Elsner, A., and Brigelius-Flohé, R. Synthetic as compared with natural vitamin E is preferentially excreted as α-CEHC in human urine: Studies using deuterated α-tocopheryl acetates. FEBS Lett 437 145-148 (1998).
4
Pope, S.A.S., Burtin, G.E., Clayton, P.T., et al. New synthesis of (±)-α-CMBHC and its confirmation as a metabolite of α-Tocopherol (vitamin E). Bioorg Med Chem 9 1337-1343 (2001).
5
Sontag, T.J., and Parker, R.S. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem 277(28) 25290-25296 (2002).
6
Birringer, M., Drogan, D., and Brigelius-Flohé, R. Tocopherols are metabolized in HepG2 cells by side chain ω-oxidation and consecutive β-oxidation. Free Radic Biol Med 31(2) 226-232 (2001).
|