| References |
| Synonyms |
|
| Formulation |
100 µg of protein G-purified IgG in 200 µl PBS containing 0.02% sodium azide |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| Specificity |
|
Background Reading
Huang, L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol 39.1 611-616 (2006).
Cress, W.D., and Seto, E.J. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol 184 1-16 (2000).
Nakayama, T., and Takami, Y. Participation of histones and histone-modifying enzymes in cell functions through alterations in chromatin structure. J Biochem 129 491-499 (2001).
Meinke, P.T., and Liberator, P. Histone deacetylase: A target for antiproliferative and antiprotozoal agents. Curr Med Chem 8(2) 211-235 (2001).
Show all 4
Hide all but first 3
| Size |
Global Purchasing |
| 1 ea |
|
Description
Antigen:
synthetic peptide corresponding to amino acids 2-17 of human HDAC3
·
Host:
rabbit
·
Application(s):
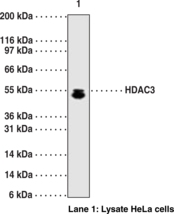
WB, IP, and ChIP
·
Histone deacetylase (HDAC) and histone acetyltransferase (HAT) are enzymes that regulate transcription by selectively deacetylating or acetylating the ε-amino groups of lysines located near the amino termini of core histone proteins.1 Eleven members of HDAC family have been identified in the past several years.2,3 These HDAC family members are divided into two classes, I and II. HDAC3 is a Class I HDAC which is related to the yeast HDAC Rpd3.4 It is primarily localized to the nucleus with ubiquitous distribution throughout human cell lines and tissues. By modifying chromatin structure and other non-histone proteins, HDACs play important roles in controlling complex biological events, including cell development, differentiation, programmed cell death, angiogenesis, and inflammation. Considering these major roles, it is conceivable that dysregulation of HDACs and subsequent imbalance of acetylation and deacetylation may be involved in the pathogenesis of various diseases, including cancer and inflammatory diseases.4
1
Meinke, P.T., and Liberator, P. Histone deacetylase: A target for antiproliferative and antiprotozoal agents. Curr Med Chem 8(2) 211-235 (2001).
2
Nakayama, T., and Takami, Y. Participation of histones and histone-modifying enzymes in cell functions through alterations in chromatin structure. J Biochem 129 491-499 (2001).
3
Cress, W.D., and Seto, E.J. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol 184 1-16 (2000).
4
Huang, L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol 39.1 611-616 (2006).
|