服务热线
021-60498804
产品中心
/ Products Classification 点击展开+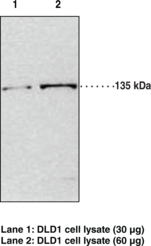
| Cat. Number | 070069138556946 |
||||||||||||||||||
| Chemical Name | JMJD2A Polyclonal Antibody |
||||||||||||||||||
| References |
Background ReadingKim, J., Daniel, J., Espejo, A., et al. Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep 7(4) 397-403 (2006). Anand, R., and Marmorstein, R. Structure and mechanism of lysine- Gray, S.G., Iglesias, A.H., Lizcano, F., et al. Functional characterization of JMJD2A, a histone deacetylase- Metzger, E., and Schüle, R. The expanding world of histone lysine demethylases. Nat Struct Mol Biol 14(4) 252-254 (2007). Marmorstein, R., and Trievel, R.C. Histone modifying enzymes: Structures, mechanisms, and specificities. Biochim Biophys Acta 1789 58-68 (2009). Show all 5 Hide all but first 3
Description
Antigen:
human recombinant JMJD2A amino acids 1-
1
Anand, R., and Marmorstein, R. Structure and mechanism of lysine- 2 Marmorstein, R., and Trievel, R.C. Histone modifying enzymes: Structures, mechanisms, and specificities. Biochim Biophys Acta 1789 58-68 (2009). 3 Kim, J., Daniel, J., Espejo, A., et al. Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep 7(4) 397-403 (2006). 4 Metzger, E., and Schüle, R. The expanding world of histone lysine demethylases. Nat Struct Mol Biol 14(4) 252-254 (2007).
5
Gray, S.G., Iglesias, A.H., Lizcano, F., et al. Functional characterization of JMJD2A, a histone deacetylase- |
||||||||||||||||||





