| References |
| Synonyms |
- OEA-d2
- Oleic Acid Ethanolamide-d2
|
| Formal Name |
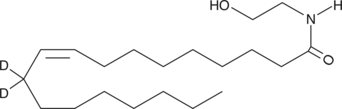
N-(2-hydroxyethyl)-9Z-octadecenamide-11,11-d2 |
| Molecular Formula |
C20H37D2NO2 |
| Formula Weight |
327.5 |
| Formulation |
A solution in ethanol |
| Purity |
≥99% deuterated product |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
CCCCCCCC/C=CCCCCCCCC(=O)NCCO
|
Background Reading
di Tomaso, E., Beltramo, M., and Piomelli, D. Brain cannabinoids in chocolate. Nature 382 677-678 (1996).
de Fonseca, F.R., Navarro, M., Gómez, R., et al. An anorexic lipid mediator regulated by feeding. Nature 414 209-212 (2001).
Fu, J., Gaetani, S., Oveisi, F., et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425 90-93 (2003).
Epps, D.E., Palmer, J.W., Schmid, H.H.O., et al. Inhibition of permeability-dependent Ca2+ release from mitochondria by N-acelethanolamines, a class of lipids synthesized in ischemic heart tissue. J Biol Chem 257 1383-1392 (1982).
Show all 4
Hide all but first 3
| Size |
Global Purchasing |
| 100 µg |
|
| 500 µg |
|
| 1 mg |
|
| 5 mg |
|
Description
Oleoyl ethanolamide-d2 (OEA-d2) contains two deuterium atoms at the 11 position. It is intended for use as an internal standard for the quantification of OEA by GC- or LC-mass spectrometry. OEA is an analogue of the endocannabinoid arachidonoyl ethanolamide (AEA) found in brain tissue and in chocolate.1 It is one of the long chain fatty acid ethanolamides that accumulates rapidly in infarcted tissue,2 but its biosynthesis is reduced in the intestine of rats following food deprivation.3 OEA is an endogenous, potent agonist for peroxisome proliferator-activated receptor α (PPARα), exhibiting an EC50 value of 120 nM in a transactivation assay.4 Systemic administration of OEA suppresses food intake and reduces weight gain in rats (10 mg/kg intraperitoneally) and PPARα wild-type mice, but not in PPARα knockout mice.3,4 These data indicate that OEA regulates food intake by a PPARα-mediated mechanism.
1
di Tomaso, E., Beltramo, M., and Piomelli, D. Brain cannabinoids in chocolate. Nature 382 677-678 (1996).
2
Epps, D.E., Palmer, J.W., Schmid, H.H.O., et al. Inhibition of permeability-dependent Ca2+ release from mitochondria by N-acelethanolamines, a class of lipids synthesized in ischemic heart tissue. J Biol Chem 257 1383-1392 (1982).
3
de Fonseca, F.R., Navarro, M., Gómez, R., et al. An anorexic lipid mediator regulated by feeding. Nature 414 209-212 (2001).
4
Fu, J., Gaetani, S., Oveisi, F., et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425 90-93 (2003).
|