| References |
| Formal Name |
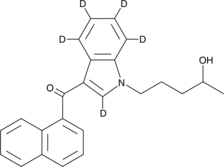
(1-(4-hydroxypentyl)-2,5,6,7,8-d5-1H-indol-3-yl)(naphthalen-1-yl)-methanone |
| Molecular Formula |
C24H18D5NO2 |
| Formula Weight |
362.5 |
| Formulation |
A solution in methanol |
| Purity |
≥99% deuterated product |
| λmax |
218, 315, 353 nm |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
O=C(C1=CC=CC2=C1C=CC=C2)C3=C([2H])N(CCCC(O)C)C4=C([2H])C([2H])=C([2H])C([2H])=C43
|
| Size |
Global Purchasing |
| 100 µg |
|
| 500 µg |
|
| 1 mg |
|
| 5 mg |
|
Description
(±)-JWH 018 N-(4-hydroxypentyl) metabolite-d5 contains five deuterium atoms at the 2, 5, 6, 7, and 8 positions. It is intended for use as an internal standard for the quantification of (±)-JWH 018 N-(4-hydroxypentyl) metabolite by GC- or LC-mass spectrometry. JWH 018 is a mildly selective agonist of the peripheral cannabinoid (CB2) receptor, derived from the aminoalkylindole WIN 55212-2. The Ki values for binding central cannabinoid (CB1) and CB2 receptors are 9.0 and 2.94 nM, respectively, for a CB1:CB2 ratio of 3.06.1 JWH 018 is one of several synthetic CBs which have been included in smoking mixtures.2,3 (±)-JWH 018 N-(4-hydroxypentyl) metabolite is a urinary metabolite of JWH 018, characterized by monohydroxylation of the N-alkyl chain.4 Its biological actions are unknown.
1
Aung, M.M., Griffin, G., Huffman, J.W., et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60 133-140 (2000).
2
Sobolevsky, T., Prasolov, I., and Rodchenkov, G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int 200 141-147 (2010).
3
Moran, C.L., Le, V.H., Chimalakonda, K.C., et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem 83(11) 4228-4236 (2011).
4
Wintermeyer, A., Möller, I., Thevis, M., et al. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem 398 2141-53 (2010).
|