| References |
| Formal Name |
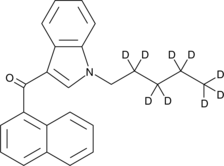
(1-pentyl-1H-indol-3-yl)-1-naphthalenyl-2,2,3,3,4,4,5,5,5-d9-methanone |
| Molecular Formula |
C24H14D9NO |
| Formula Weight |
350.5 |
| Formulation |
A solution in methanol |
| Purity |
≥99% deuterated product |
| λmax |
218, 246, 315 nm |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
CCCCCn1cc(C(=O)c2cccc3ccccc23)c2ccccc12
|
Background Reading
Aung, M.M., Griffin, G., Huffman, J.W., et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60 133-140 (2000).
Wiley, J.L., Compton, D.R., Dai, D., et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285(3) 995-1004 (1998).
| Size |
Global Purchasing |
| 500 µg |
|
| 1 mg |
|
| 5 mg |
|
| 10 mg |
|
Description
JWH 018-d9 contains nine deuterium atoms at the 2, 2’, 3, 3’, 4, 4’, 5, 5, and 5 positions. It is intended for use as an internal standard for the quantification of JWH 018 by GC- or LC-mass spectrometry (MS). JWH 018 is a mildly selective agonist of the peripheral cannabinoid (CB2) receptor, derived from the aminoalkylindole WIN 55212-2. The Ki values for binding to the central cannabinoid (CB1) and CB2 receptors are 9.0 and 2.94 nM, respectively, for a CB1:CB2 ratio of 3.06.1 Its effects on suppression of spontaneous activity, maximum possible antinociceptive effect in the tail-flick assay, and rectal temperature are comparable to those of WIN 55212-2 when tested in rats.2
1
Aung, M.M., Griffin, G., Huffman, J.W., et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60 133-140 (2000).
2
Wiley, J.L., Compton, D.R., Dai, D., et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285(3) 995-1004 (1998).
|