| References |
| Formal Name |
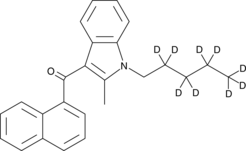
(2-methyl-1-pentyl-1H-indol-3-yl)-1-naphthalenyl-2,2,3,3,4,4,5,5,5-d9-methanone |
| Molecular Formula |
C25H16D9NO |
| Formula Weight |
364.5 |
| Formulation |
A solution in methyl acetate |
| Purity |
≥99% deuterated product |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
O=C(C1=C(C)N(CC([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])[2H])C2=C1C=CC=C2)C3=CC=CC4=C3C=CC=C4
|
| Size |
Global Purchasing |
| 500 µg |
|
| 1 mg |
|
| 5 mg |
|
| 10 mg |
|
Description
JWH 007-d9 contains nine deuterium atoms at the 2, 2’, 3, 3’, 4, 4’, 5, 5, and 5 positions. It is intended for use as an internal standard for the quantification of JWH 007 by GC- or LC-mass spectrometry (MS). JWH 007 is a potent cannabinoid (CB) receptor agonist that avidly binds to both CB1 and CB2 (Ki = 9.5 and 2.9 nM, respectively).1,2 This compares favorably with the binding of Δ9-THC, which binds CB1 and CB2 with Ki values of 41 and 36 nM, respectively.2 Similarly, JWH 007 performs comparably to Δ9-THC in murine studies on spontaneous activity, antinociception, hypothermia, and catalepsy.1
1
Huffman, J.W., Dai, D., Martin, B.R., et al. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett 4(4) 563-566 (1994).
2
Huffman, J.W., Zengin, G., Wu, M., et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists. Bioorg Med Chem 13 89-112 (2005).
|